Understanding Silane Functionalization
Alkylsilanes (silanes) have long been used for tailoring a wide variety of surfaces including silicates, aluminates, and titanates. They are currently used on an industrial scale for a wide variety of applications, such as coupling agents for glasses and polymers, as adhesion promoters, as cross-linking and dispersing agents, and for hydrophobization. Silanes readily react with surfaces through surface hydroxyl groups, or bound water to form a strongly bound coating that includes both covalent bonds and multiple van der Waals interactions.
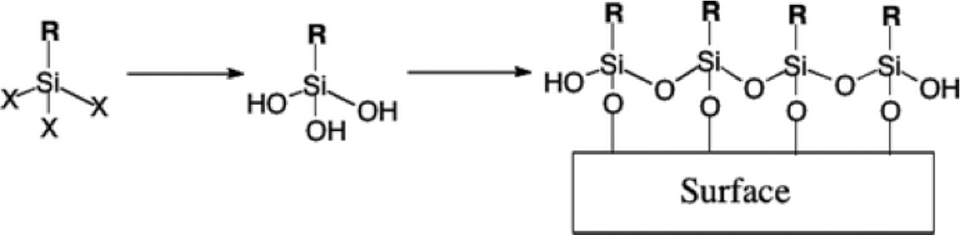
Although the exact mechanism for the formation of the surface coating is still not completely understood, it has classically been portrayed that the surface reaction of silanes takes place as shown in Figure 1. The silanes hydrolyze in solution to form silanols. In the classical mechanism, once the silanols are formed, they readily attach to the surface by their reactions to the surface OH by elimination of water. Once on the surface, these silanes polymerize internally to form Si–O–Si (siloxane) linkages.
The classical reaction mechanism leaves a number of open questions. Primarily, it does not deal with the degree of polymerization of the Si–O–Si linkages. Current characterization techniques have been unable to quantify the siloxane linkages, and it is not known whether these exist as groups of a fixed number of silanes or the entire surface consists of a continuous cross-linked structure. The relative roles of surface water or hydroxide and residual solvent moisture in the hydrolysis of the silanes is also not fully understood. Another problem that the current model faces concerns the volume constraints of alkyl chains. For a self-assembled monolayer (SAM) formed by octadecyltrichlorosilane (OTS), a typical all-trans alkyl chain has a cross-sectional diameter of 4.9 Å. Considering a typical Si–O bond length of 1.8 Å and Si–O–Si angle of 105°, the maximum available space for the alkyl chain has a diameter of 2.9 Å. Many studies have been directed at this problem, but a conclusive picture of the Si–O–Si region still remains elusive.
We have used Fourier-transform infrared (FTIR) spectroscopy to understand the structure of OTS SAMs on a silica surface, and to gain insights into the mechanism of SAM formation.
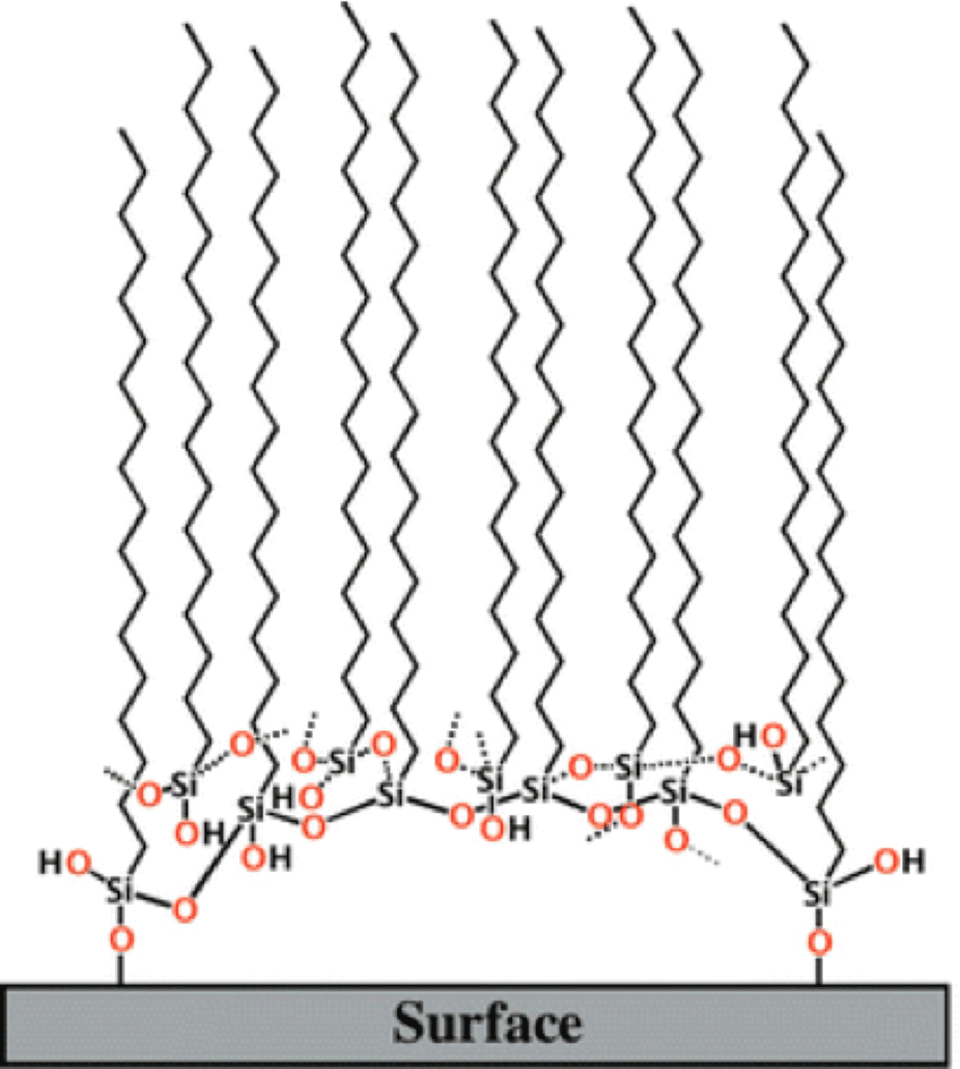
Based on our IR results we have proposed a new model for the SAM of alkylsilanes on a silica surface, as shown in Figure 2. The SAM is essentially held together by Si–O–Si linkages, and only a few terminal silane molecules actually attach to the surface. A monolayer formed by the silanes consists of a packed array of such units.
This model of mogul-like clusters of silanes may shed light on a number of previously unexplained observations and speculations.
In our lab we also work with 3-aminoisopropyltriethoxysilanes (APTES). We have shown and characterized the formation of monolayers and multilayers of APTES on SiO2 and TiO2 surfaces using different solvents.
References
- Naik, V. V.; Crobu, M.; Venkataraman, N. V.; Spencer, N. D. J. Phys. Chem. Lett., 2013, 4, 2745–2751
- Arkles, B. CHEMTECH 1977, 7, 766–778
- De Graeve, I.; Vereecken, J.; Franquet, A.; Van Schaftinghen, T.; Terryn, H. Prog. Org. Coat. 2007, 59, 224–229
- Subramanian, V.; van Ooij, W. J. Surf. Eng. 1999, 15, 168–172
- Eckstein, Y. J. Adhes. Sci. Technol. 1988, 2, 339– 348
- Maoz, R.; Sagiv, J.; Degenhardt, D.; Möhwald, H.; Quint, P. Supramol. Sci. 1995, 2, 9–24