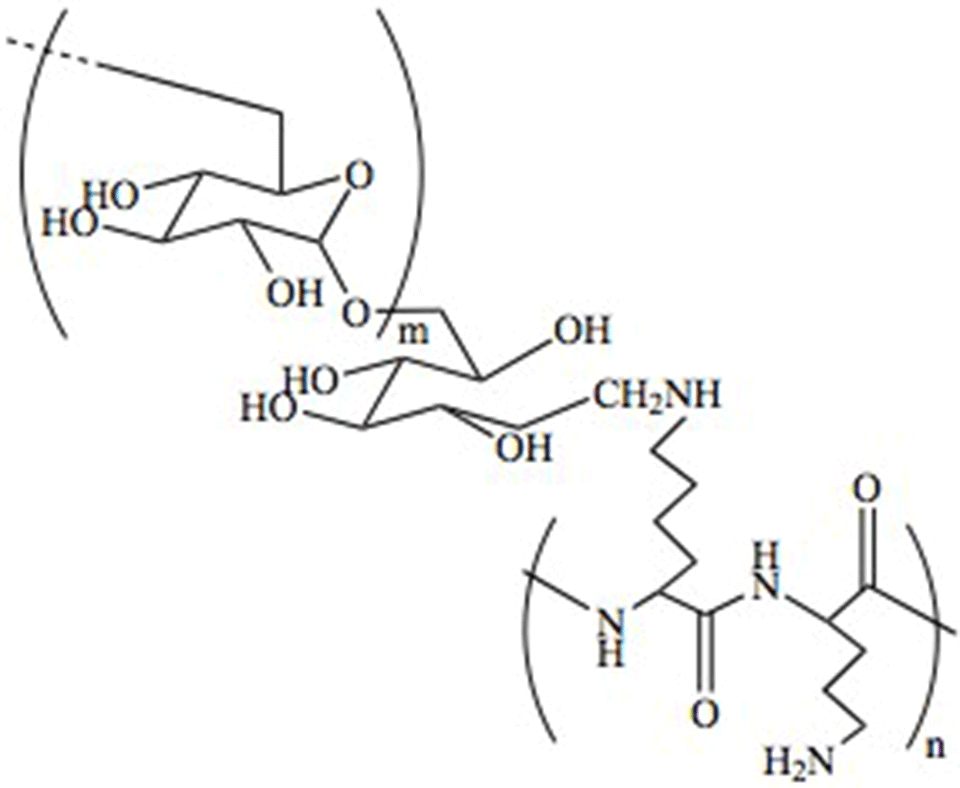
PLL-g-dextran
Poly(L-lysine)-graft-dextran (PLL-g-dex): Brush-like, Carbohydrate Chains as a Biomimetic Lubricating and Anti-fouling Coating
Poly(L-lysine)-graft-poly(ethylene glycol) (PLL-g-PEG), a graft copolymer consisting of PEG chains grafted onto a polycationic PLL backbone in a brush-like conformation, has proven to be highly effective at both preventing non-specific adsorption of proteins [external page1call_made, external page2call_made] and lubricating [external page3call_made, external page4call_made] oxide surfaces in aqueous environment. PEG, as a simple main-chain polyether, has however some limitations: it can, for instance, only be functionalized at the chain-ends and it is susceptible to thermal and oxidative degradation [5, 6]. The low long-term stability of PEG could lead to inferior lubricating properties and decreased resistance to non-specific adsorption of proteins.
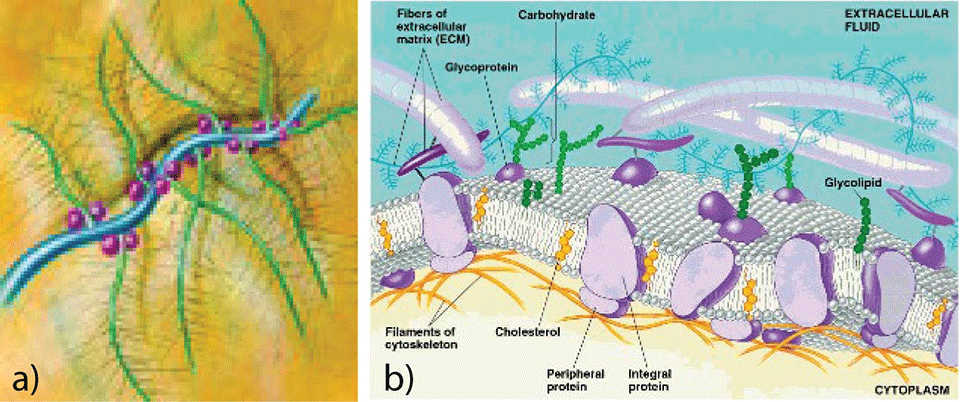
Carbohydrate-based polymer chains could represent a biomimetic alternative to PEG-based coatings for the development of anti-fouling and lubricating surfaces: sugars are in fact known to be major components of common natural lubricants (e.g. mucin, proteoglycans (Fig.1, a)) [7, 8] and of the highly hydrated glycocalyx (Fig. 1, b) which surrounds certain kinds of cells and prevents undesirable non-specific adsorption of proteins [9, 10].
The goal of the project is to develop carbohydrate-based copolymers for the functionalization of surfaces, in order to confer them with lubricating and anti-fouling properties. By maintaining the comb-like graft polymer structure of PLL-g-PEG, poly(L-lysine)-graft-dextran (PLL-g-dex) copolymers have been developed grafting dextran (dex) chains onto the same PLL backbone (Fig. 2).
The project includes:
- the synthesis of carbohydrate-based brush-like copolymers with different structures (grafting ratio, molecular weight of the backbone and of the carbohydrate side chains)
- the evaluation of the adsorption behavior and of the capabilities to prevent non-specific protein adsorption (OWLS and ellipsometry) and comparison to PLL-g-PEG
- the evaluation of the lubricating properties of the synthesized polymers and comparison to PLL-g-PEG at both the macro- (pin on disk tribometry, Mini Traction Machine measurements) and nanoscale (AFM measurements)
- the study of the influence of the architectural parameters (length of backbone, length of side chains, grafting ratio) on the lubricant and anti-fouling behavior
- the evaluation of the influence of the sugar chain hydration on the anti-fouling behavior (QCM)
- the evaluation of the stability of PLL-g-dextran coated surfaces
- the investigation of the influence of temperature and salt concentration on the lubricating and anti-fouling properties
PLL-g-dextran copolymers have been shown to spontaneously adsorb onto metal oxide surfaces, similarly to PLL-g-PEG, and to be able to greatly reduce the non-specific adsorption of proteins.
Tribological characterization revealed good performances of PLL-g-dex copolymers as boundary lubricants, both in the pure sliding and in mixed sliding/rolling contact regimes. (“End-grafted Sugar Chains as Aqueous Lubricant Additives: Synthesis and Macrotribological Tests of Poly(L-lysine)-graft-Dextran (PLL-g-dex) Copolymers”, C. Perrino, S. Lee and N. D. Spencer, submitted).
A further and eventual step towards the biomimetic lubrication will consist in the preparation of hydrogels from PLL-g-dex copolymers, by means of cross-linking. This approach might also allow to improve the mechanical properties of the coating to withstand tribological stresses (problematic area for polymer brushes, in the case of sliding between hard surfaces, due to possible polymer desorption) and the anti-fouling properties, for the higher capabilities of hydrogels to hold water layers.
References
- G. L. Kenausis, J. Vörös, D. L. Elbert, N. Huang, R. Hofer, L. Ruiz-Taylor, M. Textor, J. Hubbell and N. D. Spencer, J. Phys. Chem. B 2000, 104(14), 3298-3309.
- S. Pasche, J. Vörös, N. D. Spencer, M. Textor, Langmuir 2003, 19(22), 9216-9225.
- S. Lee, M. Müller, M. Ratoi-Salagean, J. Vörös, S. Pasce, S. M. De Paul, H. A. Spikes, M. Textor and N. Spencer, Tribology Letters 2003, 15(3), 231-239
- M. Müller, S. Lee, H. A. Spikes, and N. D. Spencer, Tribology Letters 2003, 15(4), 395-405.
- S. Han, C. Kim, and D. Kwon, Polymer Degradation and Stability 1995, 47, 203-208.
- S. Han, C. Kim, and D. Kwon, Polymer 1997, 38, 317-323.
- M. Naka, Y. Morita, K. Ikeuchi, Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 2005, 219(3), 175-182.
- R. Bansil, B. S. Turner, Current Opinion in Colloid and Interface Science 2006, 11, 164-170.
- N. B. Holland, Y. Qiu, M. Ruegsegger, R. E. Marchant, Nature 1998, 392, 799-801.
- A. Sen Gupta, S. Wang, E. Link, E. H. Anderson, C. Hofmann, J. Lewandowski, K. Kottke-Marchant, R. E. Marchant, Biomaterials 2006, 27(16), 3084-3095.
- Perrino, C.; Lee, S.; Choi, S.W.; Maruyama, A.; Spencer, N.D. external pageA Biomimetic Alternative to PEG as an Antifouling Coating: Resistance to Non-Specific Protein Adsorption of Poly(L-lysine)-graft-Dextrancall_made. Langmuir 2008, 24(16), 8850-8856