Reducing Biofouling with Polymer Brushes
Biofouling in the Marine Environment
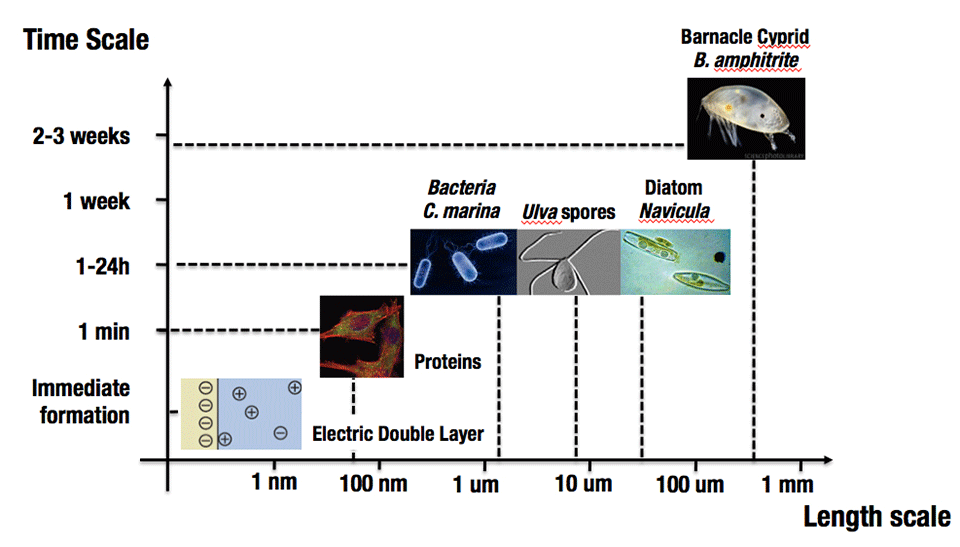
A multitude of industries operate in or near the marine environment. They all suffer consequences from the phenomenon of biofouling. Biofouling results from the immense settlement pressure of benthic organisms, which requires a solid substrate for reproduction and proliferation, as well as biofilm-forming microorganisms, such as bacteria and diatoms, which rapidly inhabit the solid-water interface [1], see Figure 1. For the shipping industry, invertebrate macro-fouling, such as barnacles, has great impact on hull drag, leading to increased fuel consumption and cost. For example, it is estimated that fuel consumption has to be increased up to 40 % for a heavily fouled hull, in order to maintain speed [2]. Further, power plants that rely on seawater-based cooling systems suffer from pipe clogging due to macro-fouling, which shortens maintenance intervals, and in severe cases, causes extended plant-shutdown periods. Aquaculture and fishing suffer from the growth of fouling on nets, which increases the need for maintenance periods and thus shortens operation time. Biofouling is ubiquitous in the marine environment, and with an estimated annual cost exceeding 60 billion USD for the shipping industry alone, has great economical as well as environmental significance.
Past and Emerging Biofouling Remedies
The use of biocides have been a successful approach to regulate fouling, and in particular tributyltin (TBT)-containing paint formulations, developed in the 1970s, were so effective at reducing fouling that no further paint development was considered necessary. However, the unspecific toxicity of TBT led to a worldwide ban on the use of these compounds in 2008 [3], which spurred research into alternative and environmentally benign biofouling solutions. Current approaches include non-biocidal and copper-containing, self-polishing paints, fouling-release coatings, and amphiphilic materials. Self-polishing paints have a slow hydrolysis rate upon water immersion, continuously regenerating the interface during their lifetime. Fouling-release coatings consist of low-modulus materials, such as poly (dimethylsiloxane) (PDMS), from which fouling can be removed upon an applied shear stress. Amphiphilic materials are co-polymers having side-chains of an immiscible nature, forming a nano-structured interface, which appears to further increase fouling-release performance combined with a non-fouling action. Apart from these industrially available strategies, research is moving towards non-fouling materials, typically consisting of surface-tethered polymers, where fouling can be inhibited in a passive manner [4, 5]. A common theme amongst these materials is that they are hydrophilic, having a strongly adhered water film that forms a kinetic barrier to fouling. They also resist fouling by steric repulsion from excluded volume effects upon compression. However, more insight is required to further understand the fouling-resistant action of these promising materials, as well as finding mechanically and chemically stable formulations that are effective towards the plethora of fouling organisms in the marine environment.
Non-fouling Polymer Model Surfaces for Controlling Fouling
To understand what happens at the biointerface between a substrate and a primary biofilm, and specifically the bonding processes and the surface properties involved, it is of the utmost importance to be able to specifically tailor the characteristics of the surface. The use of polymer brushes to do so brings many advantages due to the ease of manipulating features such as chemistry, size, surface energy, surface charge, wettability, roughness, topography, etc. [6]
In our group, poly(L-lysine)-g-poly(ethylene glycol) (PLL-g-PEG), a non-fouling model coating, has been developed, which possesses a high resistance to protein adsorption. Such behavior can be attributed to a combination of factors such as the self-assembly kinetics, final conformation and PEG surface density. PLL-g-PEG was shown to be a highly versatile product with applications in other fields besides marine biofouling, such as biomedicine, biosensing and tribology.
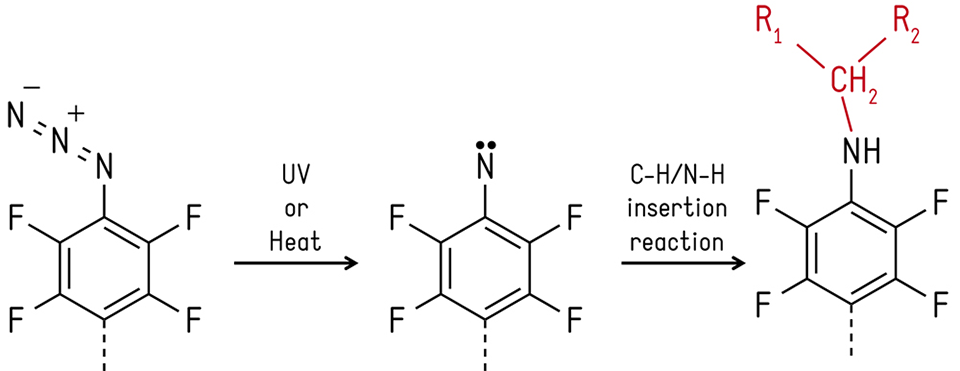
To further explore and design new coatings with enhanced performances, a novel chemical platform has been developed that successfully allows a direct comparison between polymers of different structure, size and chemistry. This technology is based on azide chemistry, which decomposes into nitrenes upon thermal or photo activation [7]. The latter species will then undergo various reactions, such as N-H and C-H insertion, allowing for any organic material to covalently bind to the nitrene (see Figure 2). Polymer-brush-like conformations using this strategy have been obtained with materials such as poly(ethylene glycol), poly(vinyl alcohol), poly(vinyl pyrrolidone), dextran, polystyrene or poly(2-ethyl-oxazoline), either in homogeneous or gradient-like surfaces. [8, 9] It was found that the level of fouling was successfully reduced on the model surfaces, however the degree of non-fouling efficiency depended on the fouling species used; protein, bacteria or macro-alga spores.
References
- Callow, J. A.; Callow, M. E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nature Communications 2011, 2, 244–10.
- Schultz, M. P. Effects of coating roughness and biofouling on ship resistance and powering. GBIF 2007, 23, 331–341.
- Sonak, S. Implications of organotins in the marine environment and their prohibition. J Environ Manage 2009, 90, S1–S3.
- Chambers, L. D.; Stokes, K. R.; Walsh, F. C.; Wood, R. J. K. Modern approaches to marine antifouling coatings. Surf Coat Tech 2006, 201, 3642–3652.
- Magin, C. M.; Cooper, S. P.; Brennan, A. B. Non-toxic antifouling strategies. Mater Today 2010, 13, 36–44.
- Senaratne, W.; Andruzzi, L.; Ober, C. K. Self-Assembled Monolayers and Polymer Brushes in Biotechnology: Current Applications and Future Perspectives. Biomacromolecules 2005, 6, 2427–2448.
- Liu, L.-H.; Yan, M. Perfluorophenyl Azides: New Applications in Surface Functionalization and Nanomaterial Synthesis. Accounts Chem Res 2010, 43, 1434–1443.
- Serrano, Â.; Sterner, O.; Mieszkin, S.; Zürcher, S.; Tosatti, S.; Callow, M. E.; Callow, J. A.; Spencer, N. D. Nonfouling Response of Hydrophilic Uncharged Polymers. Adv Funct Mater 2013, n/a–n/a.
- Sterner, O.; Serrano, Â.; Mieszkin, S.; Zürcher, S.; Tosatti, S. G. P.; Callow, M. E.; Callow, J. A. Photochemically Prepared, Two-Component Polymer-Concentration Gradients. Langmuir.2013. Submitted.